MIT and the non-existent I-129 problem
PREPENDED 2025-11-10:
This piece implies that the MIT Dept of Nuclear Engineering has done nothing to rebut Kate Brown’s anti-nuclear rants. That is not the whole truth. “A Manual for Survival” was written before Brown joined the MIT faculty and was published just about the time she did. The department apparently took the position, the book was not an MIT problem, and not worth responding to.
After joining the faculty, Brown wrote a LA Times hit piece on the San Onofre dry cask facility called “California’s San Onofre nuclear plant is a Chernobyl waiting to happen”. The NE department invited Brown to a meeting to try and educate her. She declined. The department then wrote a 3 page response signed by the whole department.
END PREPEND
This post is slightly less timid than my usual overly diplomatic pleas that we think rationally about nuclear power. Since it calls out the MIT Nuclear Engineering Department and Professor Wainwright by name, I sent a draft to MIT and her, and twice offered them the opportunity to rebut this piece. I told Wainwright I would append her rebuttal verbatim to my rant if she so desired. She came back with
Thank you again for highlighting my paper and writing an article about it. Diverse opinions will be appreciated.
In terms of the technical content, it went through a rigorous review process in Nature Sustainability, judged by experts in nuclear engineering and related fields.
The responses from the community have been overwhelmingly positive, including DOE managers. I will not add additional comments for now.
My offer is still open.
I’m so disappointed with my alma mater. Alma mater translates to nourishing mother. The nourishment coming out of MIT these days is badly contaminated milk. My first inkling of this was Kate Brown’s 2019 study of the Chernobyl release, A Manual for Survival.
MIT was once known for its hard nosed, quantitative analyses. Not fun to read, but tight reasoning grounded in fact. Here is an example of Brown’s analysis.
The ashy fallout was spreading with the winds and rain like the gray smudgy Angel of Judgement that flies through Marc Chagall’s paintings. Rather than the Hebrew characters in Chagall’s clouds, these are figures from the table of elements: a toxic brew of radioactive iodine, cesium, ruthenium, plutonium, and strontium. The angel flapped her wings and her shadow darkened a larger and larger portion of the map of Belarus, heading south and east, then north toward Chagall’s hometown of Vitebsk.{brown-2019}[p 55]
Smudgy angels can become horse riders.
The leaping, bounding, galloping rates of maladies took shape, a dark horseman riding across the Chernobyl territories.”\cite{brown-2019}[p 195]
MIT used to pride itself in getting the numbers right. Brown’s not good with numbers. She claims that half of cesium-137 will still be around for 180 to 320 years.\cite{brown-2019}[p 302] The decay half-life of Cs-137 is 30.4 years. In 180 years, more than 98% of Cs-137 will have decayed to non-radioactive barium. The biological half-life of Cs-137 is about 70 days.1 Brown: ``Radioactive isotopes do not readily leave the body”. With absolutely no support, she advances the novel theory that a chronic dose ``slow drip of beta and alpha particles ... over many years” is worse than the same acute dose.\cite{brown-2019}[p 213] Not once does she mention the repair processes with which Nature has equipped us.
I could go on and on. Imagine my disappointment when I discovered that Brown is an MIT professor. In my day, a dozen other faculty members would have immediately blasted her out of the water. She would have been the laughing stock of the whole campus.
Case in point. In the 1960’s, Timothy Leary was this endearing Harvard professor who spouted all kinds of nonsense about the medical wonders of psychedelic drugs. Needless to say, he was very popular. In 1967, he was challenged to a debate by Jerry Lettvin, a neurosurgean and neuroscientist on the MIT faculty. Before a packed auditorium, Lettvin destroyed Leary but in a friendly manner.2
But as far as I know, there was not a peep about Brown’s polemic. My disappointment turned to disgust with the entire faculty. But at least Brown is not an engineer. She is in the humanities department, which perhaps explains the prolix, florid, over the top prose.
Now I have another cause for maternal dismay, and this time it comes from within the Nuclear Engineering department. One of the more abundant fission products is iodine-129. Iodine-129 is a weak electron emitter which decays extremely slowly. I-129 has a 16 million year decay half-life. Like all iodine isotopes, if ingested it will concentrate in the tiny thyroid, magnifying the dose rate to that gland by better than a factor of one thousand. The concern with iodine-129 is thyroid cancer.
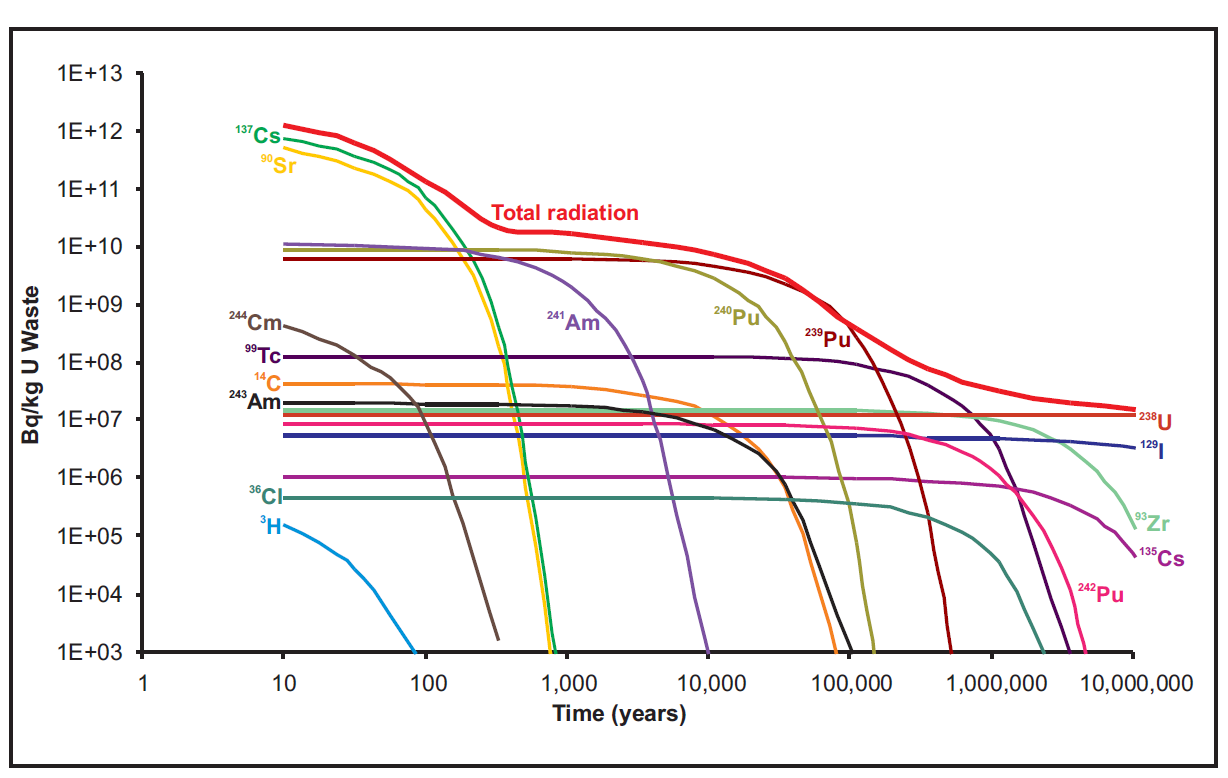
A few hundred millenia from now I-129 is a sizable portion of the remaining radioactive activity in spent nuclear fuel, and the principle photon emitter, Figure 1. So it must be a problem.
Figure 1. Long run spent fuel activity (decays per second per kg) by isotope. Don’t be fooled. The U-238 decay chain has 125 times more dose per decay than I-129.
At least MIT thinks so. Professor Wainwright of the Nuclear Engineering Department just published a study strangely titled The iodine-129 paradox in nuclear waste management strategies.\cite{wainwright-2025} If you are wondering what the paradox is, her data says diluting is better than concentrating a very slightly toxic substance. You might think that’s obvious; but Wainwright believes “Seeking perfect waste isolation should of course be encouraged”. But isolation means concentration. What we have here is a non-existent paradox inside a non-existent problem
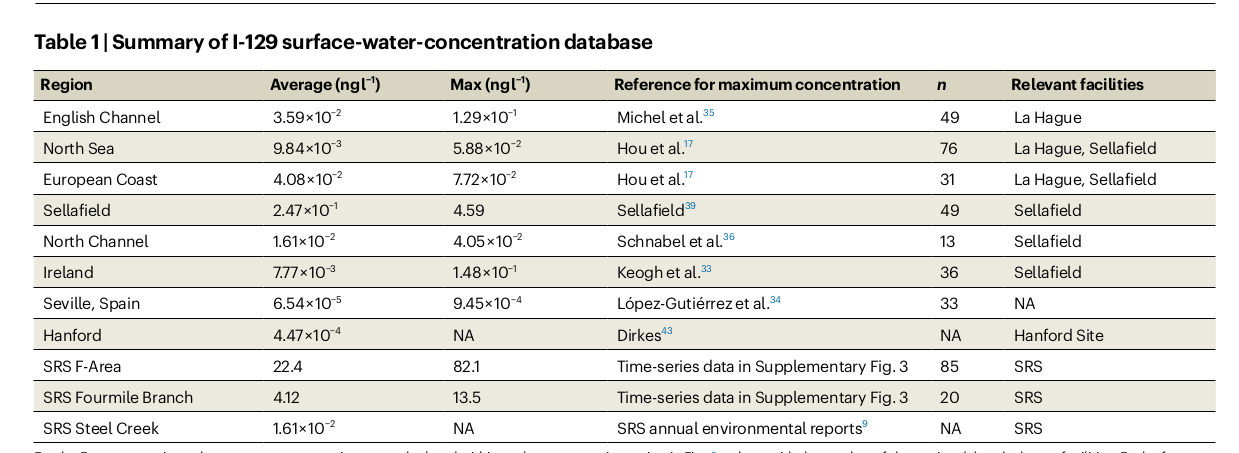
Usefully Wainwright et al give us some iodine-129 contamination numbers. Table 1 is their list of the worst of the worst.
Table 1. Worst I-129 culprits from reference \cite{wainwright-2025}.
But inexplicably the paper does not convert this contamination into thyroid dose rates. The concern is thyroid cancer, but you will not find an estimate of thyroid cancer anywhere in the paper. You won’t even find any dose rates. The word dose shows up nowhere in the paper.
If MIT won’t do the dose rate profiles, we will have to. Since no one can drink much salt water without getting very sick, I will focus on the freshwater contamination at the Savannah River Site(SRS) and Hanford. The standard but totally unrealistic assumption in all the deep geologic repository analyses is a Most Exposed Person (MEP) drinks only I-129 contaminated water for the rest of his life. In this scenario, the controlling factor is the isotope’s effective half life.3 For I-129, that’s the biological half life which the EPA puts at 80 days for iodine in the thyroid.4
As the MEP starts drinking the contaminated water, his dose rate will build up until the new I-129 intake each day is matched by the loss of I-129 in that day. At that point, his dose rate will flatten out and remain at that equilibrium level for as long as the MEP keeps up his consumption.
Wainwright et al found a spot deep in the Savannah River Site called F-Area which has a max surface water contamination of 82.1 nanograms I-129 per liter. She notes this was the result of a conscious effort to concentrate the I-129.
This is despite the fact, or ironically because of the fact, that efforts were made at the SRS to sequester radionuclides in the subsurface and within the site boundary (such as choosing the discharge location far from the rivers with a deeper groundwater table, which resulted in limited dilution.
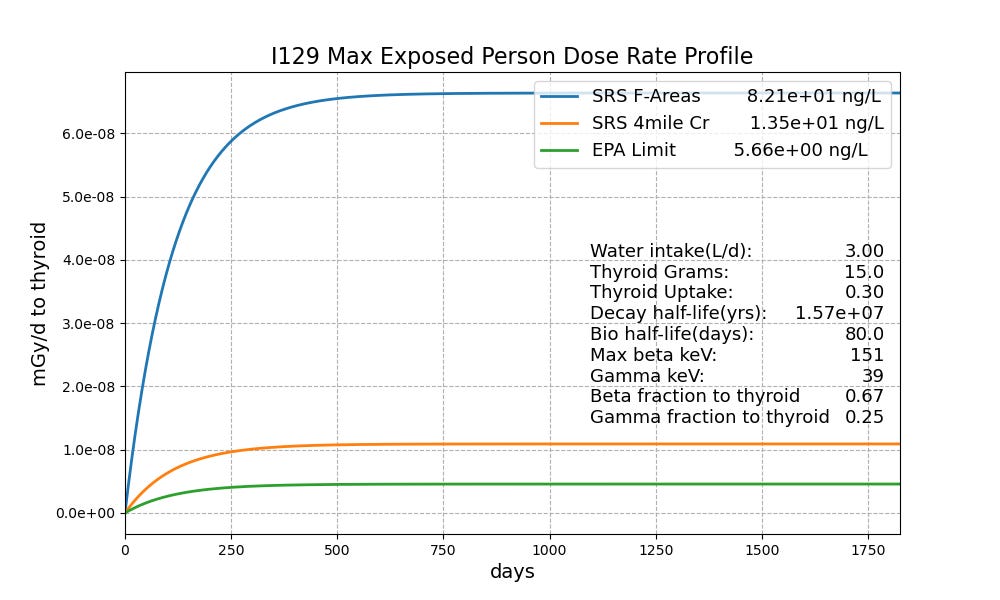
Figure 2 compares the F-Area MEP dose rate profile with the EPA I-129 contamination limit of 5.66 ng/L. It takes about 400 days to build up to equilibrium which for the F-Area location is less than one ten-millionth of a milligray per day.The average time an I-129 atom spends in the thyroid is 114 days. The probability of it decaying in that period is 0.00000002. Long half-lives beget teeny-weeny dose rates.
Figure 2. MEP I-129 dose rate profile for Wainwright’s worst contamination
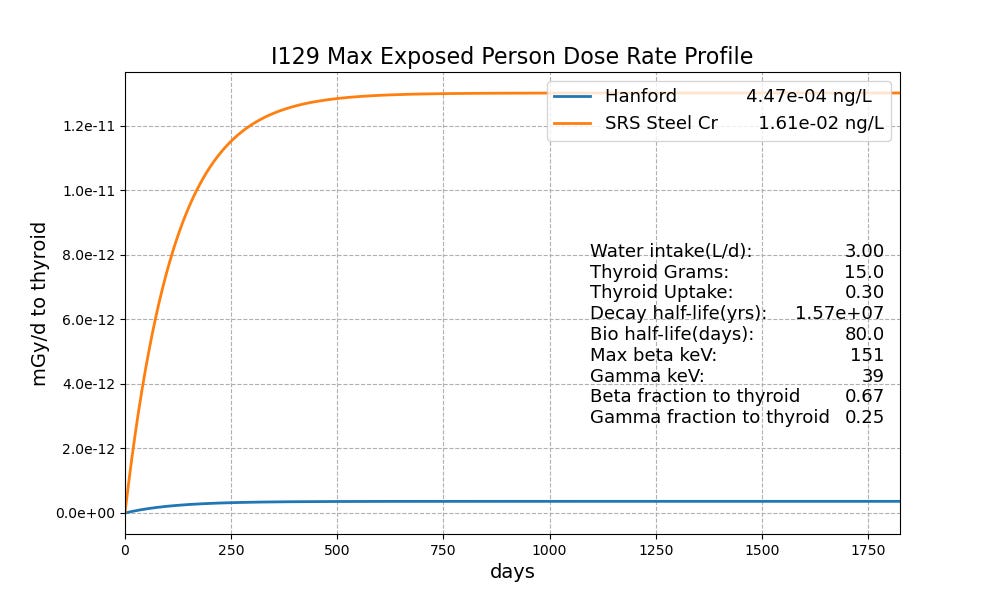
For the public, it just gets sillier, or far more accurately, more tragic. For the Steel Creek Site on the SRS boundary, the equilibrium MEP dose rate is down more than another factor of 1000, Figure 3. The Hanford Reservation is the site of a ``clean up” program projected to cost half a trillion dollars, perhaps the biggest boondoggle ever. In the Columbia River just downstream from Hanford, we are down yet another factor of a million. The Hanford strategy of dumping the I-129 into the Columbia is far less risky than the SRS strategy of concentration. Dilution is the solution.
Figure 3. MEP I-129 dose rate profiles for worst sites to which the public has access
But for I-129 neither strategy is a problem. Under SRS’s stupid strategy, the equilibrium F Area MEP dose per day is less than one thousandth of a banana dose, and that’s only to the tiny thyroid. The 40 year LNT mortality for this MEP profile is 0.000000096 (9.6e-8). The SNT mortality is 0.00000000000000000069 (6.9e-19). When I call I-129 a non-existent problem, I mean it literally. All this should be well known to a Nuclear Engineering Department. Kate Brown has the ignorance defense; Haruko Wainwright does not.
Just because something is radioactive does not make it dangerous. Bananas are not dangerous. Brazil nuts are not dangerous. Humans can be very dangerous; but it’s not because, like every large living organism, we are radioactive. The smart people in Nuclear Engineering at MIT know this. I can’t be sure why MIT chose to “analyze” I-129 disposal without calling attention to the negligibly minuscule dose rates. But with friends like this, nuclear power does not need any enemies.
Biological half-life is a measure of how rapidly the body rids itself of a substance in the normal course of events. In this case, 50% of the absorbed cesium will be gone every 70 days.
Both Leary and Lettvin were characters. Lettvin was this big, fat, jolly guy; but his wife Maggie was a fitness freak who had the most spectacular body you can imagine. She taught yoga or something on campus. When she walked through the gym in her tights, all the pick up games would grind to a halt so we could ogle her. Those were fun times.
A radioactive isotope absorbed into the body can leave the body both by decay and by biological processes. The effective half-life is the combination of these two processes. For I-129, the decay half life is so much slower than the biological half-life that the effective half life equals the biological half-life. For the geeks, half-lives combine like parallel resistors.
EPA’s number is a conservative average. I’ve seen numbers as low as 30 days. It depends in part on the condition of the thyroid and how abundant iodine is in the individual’s diet.


I have seen a lot of people worry about the radiation hazard of depleted Uranium to populations.
I could care less - Uranium is quite toxic as a chemical. The chemical hazard of Uranium to people is far far higher than its radiation hazard, enough so that any sane risk calculation would ignore the risk contribution from the radiation - the radiation portion of the risk is far smaller than the uncertainty in the associated chemical hazards.
I was a safety officer in a chemical process lab > 50 years ago. I remember a meeting where a very old bottle of a particularily dangerous chemical was found on a shelf. Nobody knew about it. One chemist was worried that it was a carcinogen. The old expert's comment was - 'who cares, that stuff is classified as a chemical weapon. It will kill you long before you get cancer from it."
In terms of risk to life, property and large scale destruction, I worry about dam failures.
Similar sort of misinformation going on in Canada, no surprise.
It comes mostly from perennial antinuke activist Gordon Edwards, who frequently presents himself as Canadian Coalition for Nuclear Responsibility (CCNR).
He regularly receives "intervenor" funding from the CNSC - Canadian Nuclear Safety Commission - either directly or by contract from various antinuke groups, such as CELA (Canadian Environmental Law Association).
In recent years, Edwards has been campaigning against Canada’s project for used CANDU fuel disposal in a DGR (Deep Geological Repository), which is now slated to be built in western Ontario, about 30km west of the town of Ignace – which applied to participate in the site selection process 15 years ago, and voted overwhelmingly in favor of “hosting” the project early last year (2024).
Needless to say, Edwards’ scare mongering includes “the I-129 problem” – see linked picture below, which is a screen grab from a YouTube video from one of Edwards’ presentations last year.
Edwards also includes “the Cs-135 problem”.
https://cdn-images-1.medium.com/v2/resize:fit:1500/1*oG8Uka43J0Z_98ld_xYXqQ.png
My comments on Edwards’ claims were as follows:
First of all, it’s rare that nuclear fuel bundles have so much as a pinhole defect: When one does, it is immediately detected in the reactor, and the defective fuel bundle is removed.
Secondly, the thermal conditions inside the reactor are pretty fierce, with 300°C and high-pressure, high-velocity flow.
Once the defective fuel bundle is removed and placed into the cooling pool for storage, it stops leaking, because the thermal conditions are benign.
Used nuclear fuel bundles are barely warm, after 50 to 70 years of surface storage, whereas the boiling point of Iodine is 184.3°C, and for Cesium the boiling point is 671°C.
Perhaps more importantly, Edwards is talking about the extremely long-live isotope Iodine-129, not the short-lived isotope Iodine-131.
Iodine-131 is of concern in reactor operation, but NOT in long-term storage: It is completely gone in a few months following removal from the reactor. Iodine-129 remains in the used fuel for a very long time, but its radioactivity is extremely low: Canada’s nuclear regulator, the CNSC, requires prophylactic iodine pill distribution in the vicinity of nuclear power plants, but does NOT require their distribution around long-term used fuel storage sites, because the remaining Iodine-129 is insignificant, and there is no possibility of a million used fuel bundles getting pulverised while in storage – at least not until the next ice age, with the advancement of thick glaciers.
Third, let’s not forget – as Edwards does every time – that used CANDU fuel is 99% natural uranium, with just 0.6% fission products.
Out of that 0.6% only a small fraction are volatile species like Iodine-129 (I129).
Most is trapped in the ceramic pellet crystalline structure, with a small fraction available for release if the fuel were damaged during a transport accident.
Each fuel bundle is comprised of ceramic uranium fuel pellets, containing 19.2kg of Uranium, inside 37 zircaloy tubes that are welded shut (ie. half a kilo of uranium pellets in each tube).
After 50 years of storage, prior to shipment to a DGR, the amount of I129 in used fuel is 0.29 MBq/kgU (Where “MBq” stands for Mega-Becquerels or millions of Becquerels, and is listed per kilogram of Uranium (kgU) initially in the fuel bundles).
For comparison, the human body contains about 0.008 MBq of radioactivity, more than half of which is natural Potassium-40 (K40).
The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) use a dose conversion factor of 0.044 mSv/MBq for I129.
So, if ALL the I129 in a kilogram of used fuel were released and 100% of it absorbed by nearby people, the radiation dose they would receive (in milli-Sieverts, or “mSv”) would be 0.29 MBq/kgU x 0.044 mSv/MBq = 0.013 mSv (collective dose, not individual dose).
For comparison, the typical annual radiation dose Canadians get from natural background sources is between about 1.2 and 4 mSv, or roughly 150 times more (depending on where in Canada you live).
Of course in reality, only a tiny fraction of the I129 in that kilogram of used fuel could be released, and it’s extremely unlikely that anywhere near 100% of that would de absorbed by nearby people (or anything else).
Lastly, let’s remember that Iodine-129 occurs naturally in trace quantities in the environment, because uranium is ubiquitous in the ground and in oceans and lakes:
Somewhat like Radon gas, I129 comes from uranium, albeit in somewhat different ways.
While Radon is simply a decay product of uranium, I129 is produced by spontaneous fission of uranium, as well as fission due to neutrons in cosmic rays hitting uranium atoms.
---------------------
PS. Readers here are invited to invited to join my “Rad Toolbox” group on Facebook: https://www.facebook.com/groups/150981218802374