An earlier version of this piece was posted on Works in Progress. Apologies for double posting, but I wanted to give the Gordian Knot subscribers a chance to comment.
Take Away
For practical purposes, spent nuclear fuel remains radioactive forever. However, the penetrating form of radiation is essentially gone in 600 years. After that, the used fuel must be swallowed to be harmful. But that would require eating rocks. Bacon is probably more dangerous than aged nuclear fuel. You are much more likely to swallow that carcinogen.
Precis
Recent events have forced all sorts of people to think seriously about nuclear power, many for the first time in their lives. And often their first question is: what about the waste? What will we do with the extremely radioactive spent fuel?
There are two keys to understanding the nuclear used fuel (aka waste) problem:
1) The quantities involved. Thanks to nuclear's amazing energy density, the amount of used fuel is so small that we can afford to handle it very carefully.
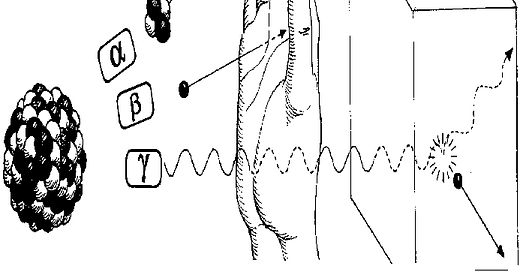
2) The difference between the three forms of radiation emitted by the used fuel: alpha particles, electrons, and photons (often called gamma rays). Alpha particles have no penetrating power. They are stopped by a piece of paper or a few centimeters of air. Electrons (confusingly called beta rays in this context) have very little penetrating power. Most are stopped by the outer layer of our skin. Alpha particles and most electrons must be swallowed to be a health hazard. They require little or no shielding.
Photons on the other hand can have enormous penetrating power. High energy photons can pass all the way through a human being. You don't want to mess with these photons. Fresh used fuel puts out a lot of high energy photons and needs lots of shielding.
Overtime, radioactive materials decay, and the radiation levels drop off. Different radioactive materials decay at very different rates. Most of the photon emitters in the used fuel decay rather rapidly. After less than 600 years, the photon dose rate at a fuel element surface is so low, that according to Department of Energy rules, it can be handled without any shielding at all. For practical purposes, the photon emitters are gone in 600 years. What's left are alpha and a small amount of electron emitters.
The electron and especially the alpha emitters tend to decay far more slowly. 95% of the used fuel is Uranium-238, an alpha emitter. The half-life of U-238 is 4.5 billion years. The alpha emitters are around essentially forever. So the rule is simple. Don't eat spent nuclear fuel, even if it's 600 years old. But you have plenty of substances around the house for which the same rule applies.
This is what we are worried about?
Figure 1 shows the dry cask storage facility for the Connecticut Yankee nuclear power plant near Haddam Neck on the Connecticut River.
Figure 1. Connecticut Yankee Dry Cask Storage Facility
Connecticut Yankee (CY) was a 619 MWe pressurized water reactor which ran for 28 years between 1968 and 1996. During that time the plant produced 110 million MWh. There are 43 casks on a concrete pad, 70 feet by 228 feet. These casks contain about 1020 tons of used fuel. The fuel is surrounded by 3.5 inches of steel and then 21 inches of reinforced concrete. Each cask weighs about 126 tons, of which about 25 tons is the used fuel itself. Each cask also has internal passages for natural draft circulation to remove the heat produced by the used fuel's radioactive decay. These show up in Figure 1 as the rectangular slots at the bottom and top of each cask.
If Connecticut Yankee had been a coal plant, it would have produced between 3,000,000 and 6,000,000 tons of toxic ash in its operating life, not to mention 110 million tons of CO2. If we attempted to store this ash on the CY fuel cask pad, we would have a column of ash about 7000 feet high. The volume of solid waste per unit power produced by a nuclear power is 100,000 times less than that produced by a coal plant.
The used fuel in these casks falls into one of three categories:
1) Uranium
2) Plutonium and other transuranics.
3) Fission products.
Uranium
By weight, uranium represents about 96% of the used fuel. Almost all of this is U-238. This means the fuel is potentially quite valuable. Current nuclear's energy density is 500,000 times higher than fossil fuels, 12,500,000,000 times higher than hydro, and 250,000,000,000 times higher than wind, when the wind is blowing. That sounds pretty good. But it could be a lot better. Today's nuclear technology is woefully inefficient because it can't burn U-238. More than 97% of the energy that could theoretically be generated from the fuel is still in the fuel when it is pulled from the reactor, Figure 2.
Figure 2 Composition of Used Fuel. It’s mostly Uranium-238.
Transuranics
Some of the neutrons bouncing around in the reactor do not result in fission, but are absorbed by the fuel, transmuting the uranium into still heavier elements, mostly plutonium. This group is known as the transuranics (TRU). By weight the TRU represent about 1% of the used fuel.
Transuranics decay by emitting alpha particles and some electrons. Neither have any real penetrating power. Few can penetrate the outer layer of skin. In order for alpha particles or electrons to be a problem, they must be ingested or inhaled. Being a bit rhetorical here. To be precise, the alpha and electron emitters must be swallowed for the particles to be harmful.
Transuranic decay tends to be very slow with some important transuranic isotopes having half-lives of the order of 100,000 years. Transuranics also can be quite valuable. Some such as Plutonium-238 can be used to power deep space probes. Others can be processed into excellent nuclear fuel, although currently this is not quite economic, in part because the fission product decay makes handling the used fuel so difficult.
Fission Products
Fission products are the result of the nuclear fuel splitting into two pieces. They represent about 3% of the used fuel. Most fission products decay by emitting photons and electrons. It is the photons that makes used fuel difficult to handle. A high energy photon can penetrate deep into a human body. Photons are the reason the casks in Figure 1 are as big as they are. They provide the shielding that allows essentially unlimited access to the storage facility. Cask hugging, Figure 3, is unlikely to become a national sport. But with a tiny extra bit of money, the casks could be turned into climbing walls or the pad turned into a paint ball court.
Figure 3. Cask Hugging at Palo Verdes. Credit: Paris-Ortiz-Wines.
Figure 4 puts some numbers on the decay process. Dose is the amount of radiation energy absorbed by our tissue. Dose is measured in joules per kg of tissue. Gray is a shorthand name for joules per kg. The graph is in milligrays per day (mGy/d). The key feature of Figure 4 is that photon decay is relatively rapid. By year 600, 99.999% of all the photon emitters are gone, relative to the end of year 1. In fact, the photon dose rate becomes so low that, according to US Department of Energy rules, the used fuel elements can be contact handled, handled without any shielding at all. After year 600, the spent nuclear fuel must be swallowed in order to do any damage.
Figure 4. Dose Rate at Fuel Element Surface, based on Canadian Waste Management Organization, Used Fuel Repository Design, TR-2012-16, Figure 5-13. The vertical scale is logarithmic.
The swallower's main problem would be uranium's chemical toxicity. Only 1% of the fuel is plutonium, and 99.997% of the plutonium will be excreted in a day or two. About 20% of the uranium will be absorbed into the blood and about 70% of that will end up in the kidneys. Ingesting 5 grams of uranium over a short period will result in a 50% chance of death due to kidney failure.1 Cohen calculated that it would take 225 grams of 600 year old fuel to give you a 50% chance of fatal cancer from the radiation.2
But for how long?
It's all very well to talk about dry cask storage; but there's got to be an end to it. How long do we have to keep the stuff around?
To answer that question, I need to draw your attention to a looming problem. Currently we have roughly 6.1 million tons of reasonably assured uranium reserves. If the planet were to be totally powered by nuclear using conventional reactors, these current reserves would last less than 5 years. While more uranium will be found, clearly this is not sustainable.
Fortunately, a solution exists: breeder reactors. Breeder reactors are not new. They go back to the dawn of the nuclear age. The Russians have had a 600 MW breeder operating since 1980 and an 800 MW breeder since 2016. China and India have active breeder programs. A breeder reactor can burn not only U-235 but also U-238. This will extend our uranium reserves by a factor of 140.3 Then we would have at least 700 years before we need to dip into thorium and low grade uranium ores.
Right now in the USA alone, there is about 90,000 tons of already refined U-238 sitting in dry cask storage around the country. The stupidest thing we could do is put this fuel where we cannot get it. Of course, that's precisely what we are planning to do, in the form of deep geologic repositories, the equivalent of throwing diamonds into a volcano.
Figure 5. Histore Pad. 580 tons of used fuel per acre
Rather than this criminal idiocy, the obvious solution to the nuclear waste problem is:
1) Keep the material in dry cask storage until the photon emitters have mostly decayed away, at most 600 years. If the US were to generate all its electricity from nuclear, a 600 year decay time means she would have to devote at most 21 square miles --- about the size of Manhattan --- to dry cask storage. This is based on the Histore system, Figure 5, which needs one acre to store 580 tons of used fuel.
2) Extract the valuable medical and industrial isotopes.
3) Extract the remaining uranium and transuranics and burn them as fuel in breeders. This will reduce the waste volume by about a factor of 15. Far more importantly, it will extend our uranium reserves by a factor of 140.4
4) The remaining material will be low level waste, pretty innocuous. It can be diluted and landfilled.
Once we recognize the difference between penetrating and non-penetrating radiation, 600 year old spent nuclear fuel becomes just another poison.
Kathren, R. and Burklin, R., Acute Chemical Toxicity of Uranium, Health Physics, Vol 94, No 2, 2008.
Cohen, B., The Disposal of Radioactive Wastes from Fission Reactors, Scientific American, Vol 236, No 6. June, 1977
A good conventional reactor can generate 5.2 terajoules from a kilogram of 3.8% enriched uranium. It takes 9.5 kg of mined uranium to produce that enrichment. A breeder can produce 78 terajoules from a kilogram of mined uranium.
Because the material is so valuable, it will almost certainly pay to do the extraction well before all the photon emitters have decayed away. The residue will need to be returned to storage until it can be handled without shielding.









Hi, Jack - My co-author Tim Maloney and I, in our upcoming book Fear of a Nuclear Planet, have determined how many solar panels it would have taken to produce the same energy as the "spent" fuel stored in the Connecticut Yank dry cask storage farm.
Using the Sunpower E-327 panel with an estimated 40-year lifecycle, we calculate that on the same sized storage pad, the used panels would form a solid stack 670 feet high, or 530 feet high if the panels were crushed flat. I'll send you the graphic and calculations by email -- feel free to use it!
“It’s only dangerous if you eat it”. At longer time scales, should we worry about inhaling wind born dust particles created by erosion (or explosion)? I.e. eating by other means.
If 600 years is the relevant timeframe for nuclear waste, why do we hear about thousand-year duration nuclear wastelands, particularly for Chernobyl? Did that disaster create compounds that are more dangerous and long lived than standard nuclear waste?