Eben Byers and Radithor
Bad error in Figure 1 caption fixed.
Figure 1. Three grams 1 micro-gram of Radium-226 (1620 year half-life) spiked with an equal amount of Radium-228 (5.7 year half-life) in terms of decays per second.
The sad case of Eben Byers gives us an upper bound on the dose rates our bodies can handle. In the early 20th century, Byers was a wealthy socialite, ladies man, and excellent athlete. He was the 1906 USA Amateur golf champion. Returning from the Yale-Harvard game in 1927, he fell from his Pullman berth, and injured his arm. The arm failed to heal properly.
So in January, 1928, his doctor proscribed Radithor, a nostrum containing radium, whose ``inventor”, William Bailey, claimed would cure just about any medical problem. Each bottle contained radium putting out about 75,000 decays per second. Byers immediately started feeling better and quickly increased his uptake to more than three bottles per day. At a dollar a bottle (about twenty 2025 dollars), this was an outlay that only the well off could afford.
In 1930, Byers started experiencing blinding headaches and terrible toothaches. In December, 1930, he stopped taking Radithor; but by that time he was deteriorating rapidly. His teeth fell out. His jaw just crumbled away. He died a miserable death in 1932. The Wall Street Journal ran an article with the headline “The Radium Water Worked Fine Until His Jaw Came Off”.
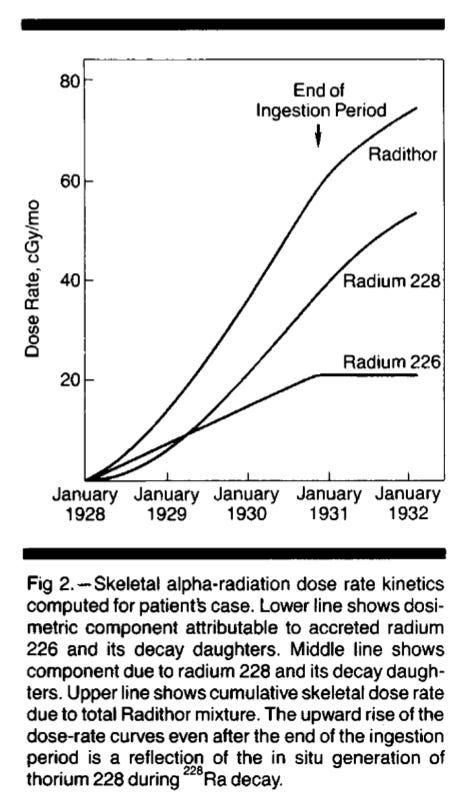
Figure 2. Byers’ Dose Rate Profile
In 1965, Byers’ body was temporarily exhumed and studied. Macklis et al used the results to estimate his dose rate profile, Figure 2.\cite{macklis-1990}[p 621] Byer’s dose rate quickly built up to 15 mGy per day. Since the dose was almost all alpha particles, this is close to 300 mSv/day. This dose rate overwhelmed Byers’ DNA repair systems.
Macklis et al reckon that, by the time Byers died, he had received a total absorbed skeletal dose of 366,000 mSv. 360 sieverts is 50 times the lethal acute dose. The authors take this in stride, commenting ``It is surprising that the patient remained asymptomatic until late in 1930.”1 Actually, if LNT is valid, it is impossible.
The Other Radithor Drinkers
Eben Byers was not the only person who took Radithor. Bailey sold some 400,000 bottles of his product between 1925 and 1930. Robley Evans estimates that Bailey had more than a 1000 customers for his concoction. Evans was able to track down about 200 cases of very high radium doses and study them intensely, including xraying their bodies.
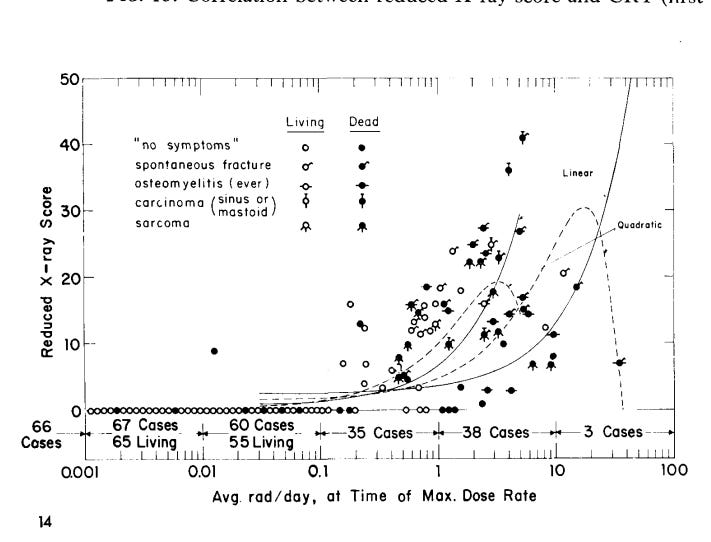
Figure 3. Evans Radithor Drinkers. 0.1 rad/day (1 mGy/d) of radium is about 20 mSv/d.\cite{evans-1966}[Fig 14] There’s an awful lot of work behind this rarely publicized figure. Study it carefully.
When he plotted the results against the maximum dose rate they had received, Figure 3, he found no clinical symptoms in the subjects whose dose rate had peaked at about 4 mGy/d or below.\cite{evans-1966} However, a few of the less than 4 mGy/d xrays showed abnormalities down to about 1 mGy/d.2 For a nearly all alpha particle dose, these Gy numbers correspond to close to 80 and 20 mSv/d respectively. Strong evidence that our bodies can handle dose rates up to at least 20 mSv/d.
The fraudster Bailey partook of his own product. When his body was exhumed, Rowland found he had consumed roughly one-third as much Radithor as Byers.\cite{rowland-1994} William Bailey died at 64 of bladder cancer, probably unrelated to the Radithor. Did I mention that both Byers and Bailey were Ivy League products, Yale and Harvard?
Figure 2 is unique in my experience. It is the only explicit dose rate profile that I have found in the literature prior to the GKG’s reviving the concept. Perhaps the choir knows of other examples. Figure 3 shows that others were aware of the importance of dose rate; but it too is a rarity.



Three grams of Ra-226 puts out a hell of a lot more than 75000 Bq. Kind of surprised at this statement as this isotope is the easiest of them all to calculate this for.
That Byers did not die after swallowing 50 times the total lethal dose is obvious: the dose rate is the major factor. Byers died of a fulminating cancer. But most cancers take about 10 years to appear after exposure, and the risk is random. It is therefore unwise to conclude from the observations that 1 mGy/day is safe.