Uranio o Plomo
Which is worse, Uranium or Lead?
Error: fixed bad mistake on Byer’s consumption of radium
Figure 1. Do not feed him either.
On several occasions, I’ve made the cavalier comment that aged (~500 year old) spent nuclear fuel is roughly as dangerous as lead. Time for some fact checking.
The Nuclear Reorganization Act envisions a future in which we keep the spent nuclear fuel in dry cask storage until the photon emitters, most importantly Cesium-137, have decayed away to the point that the material can be handled with little or no shielding. This will be at most 600 years. At that point, the penetrating radiation is effectively gone. The remaining heavy metals will have to be swallowed to be a health problem. We then extract any valuable material, most of which will be recycled back to the plants as fissile or fertile fuel. Lather, rinse, and repeat. But over time the unusable isotopes will build up. What do we do with the stuff that is not economically recoverable?
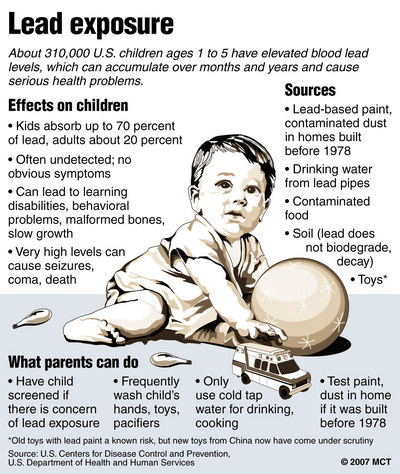
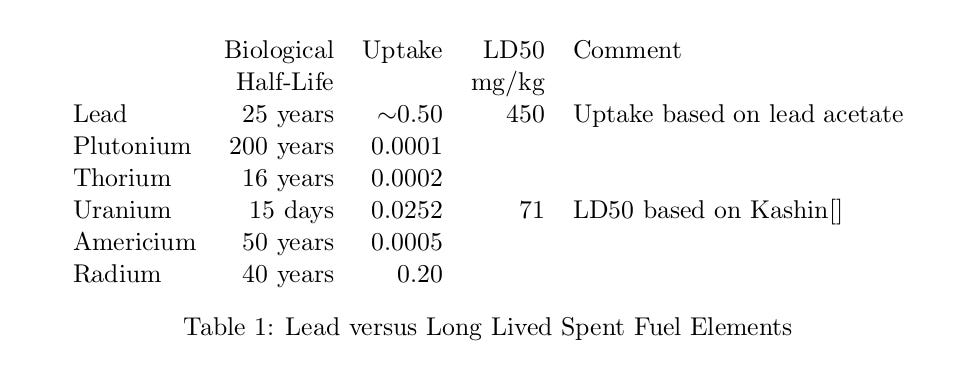
All heavy metals are poisons. The one we are most familiar with is lead, Figure 1. Table 1 compares the long lived, spent fuel elements with lead. I’ve also thrown in radium, even though it is not a spent fuel component.
Uptake is the fraction of the ingested material that is absorbed into our blood stream. The unabsorbed material will be excreted in a day or two. Thorium and all the transuranics have nearly zero uptake. This is due to their insolubility. All the isotopes of these elements have multi-decade decay half-lives, most far longer. They are not a health problem, because they go right through us.
Radium has a fairly high uptake and a long biological half-life. We know quite a lot about radium’s dangers thanks to the radium dial painters and Radithor. If you drink 6 grams 2 micrograms of Radium-226 every day for a couple of years, you will die a horrible death as Eben Byers did.1
Regardless of what William Bailey claimed, radium should not be imbibed. However, radium is not produced in a nuclear reactor. There is no radium in fresh spent fuel nor in a nuclear power plant release. Radium will be produced by the decay of uranium (and any thorium) in the fuel; as it slowly builds toward its equilibrium concentration. For Ra-226 in U-238, this is 0.000034%. Radium will always be a minuscule fraction of the discarded material. Radium is a non-issue for spent nuclear fuel.
That leaves us with uranium. Uranium has a worrisome uptake and concentrates in the kidney. Kathren and Burklin estimate that consuming 5 grams of uranium in a short time will result in a 50% chance of death due to kidney failure.\cite{kathren-2008} The good news is that uranium has a short biological half-life. Biological half life measures how long it takes the body to get rid of absorbed material in the natural course of events. In uranium’s case, half of any absorbed material will be urinated out every 15 days. This combined with long decay half-lives means uranium’s chemical toxicity is much higher than it’s radiotoxicity.2
The uptake of lead depends on its form. If you swallow a fishing weight, which kids occasionally do, the uptake will be very low. Swallowing lead pellets from shot game is much more likely, and has about the same result. However, some common lead compounds have extremely high uptake. Lead acetate is the most famous. Roman wine was laced with lead acetate. Some historians blame the erratic behavior of some Roman emperors on their consumption of lead.
Lead serves no useful biological function; but it is able to screw up a lot of those functions. Most importantly, it messes with our brains. And the long biological half life mean small chronic doses will build up in the body. And then a whole lot of bad things start to happen.
If we are talking about swallowing a lot of material in a short time, uranium is somewhat more dangerous than lead. If we are talking about consuming small amounts more or less regularly, lead is clearly more dangerous than uranium. Both should be kept out of our diet.
Economically unrecoverable lead is disposed of by labeling it as hazardous waste. What this means depends on the state and local community. The basic requirement is the waste must go to a licensed landfill. Licensed landfills are usually required to have a groundwater monitoring system, and do regular reporting of the measurements. This is done by sampling the water from a grid of wells in and around the landfill. There is no health argument for treating a mixture of uranium, thorium and transuranics much differently.
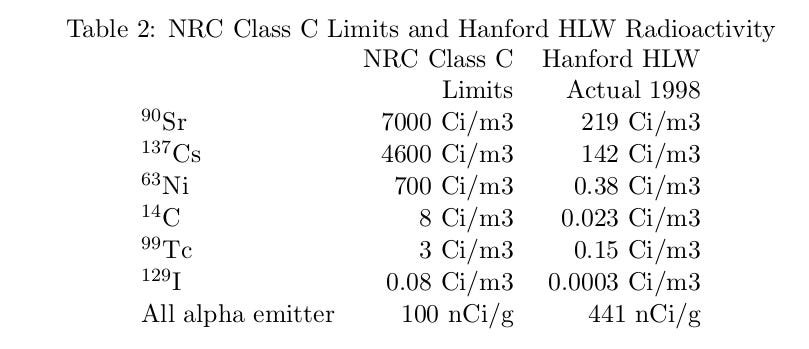
Currently, the NRC allows landfill disposal for waste meeting their Class C limits, Table 2. But by fiat, spent fuel cannot be Class C even if it meets all the Class C requirements???? The Nuclear Reorganization Act ends this senseless distinction. The radiotoxicity of any material depends on its current composition, not where it came from.
Figure 2. The Barnwell Class C Landfill. Nothing really special.
The requirements for a Class C nuclear waste landfill are little more than a strict definition of a hazardous waste landfill. Figure 2 shows the Class C landfill at Barnwell, South Carolina. The monitoring and reporting requirements include rules for sampling the groundwater radioactivity regularly. Even a tiny amount of radiation is ridiculously easy to measure; and each isotope gives off its own frequency signature. If there is any problem at Barnwell, we will know about it.
To meet the Class C requirements, it will probably be necessary to dilute the spent fuel. Darryl Siemer argues for a simple process in which the spent fuel is vitrified into marbles of iron-phosphate glass, using the same machine that makes toy marbles. The marbles then become the aggregate in a cementitious grout. For spent fuel that has been aged a few hundred years, it will not take a lot of dilution. Table 2 shows that in 1998 the Hanford spent fuel easily met all the NRC requirements for Class C except for the non-penetrating alpha emitters, even though it was less than 50 years old.
I have no idea how NRC came up with a 100 nCi/g, for something that must be swallowed to be a problem; but for the moment let’s accept that number. All that would be needed is 4.4 grams of glass and grout to gram of spent fuel. It’s true that the Hanford spent fuel is not a good proxy for commercial spent fuel, mainly because the fuel was extracted from the reactor after something like 60 days of operation, well before the long lived fission products, most importantly Cs-137, had a chance to build up. But that just means we will have to wait longer than 50 years.
A monitored landfill is not only much cheaper than a deep geological repository; but it also has the big advantage that, if anything goes wrong, we will know about it and can fix things. And when the material once again becomes valuable, at it it eventually will, it will be easily recoverable. Did you know that the fraction of U-235 actually grows through time as the transuranics decay?\cite{ornl-tm-13170}
Actually, he drank the equivalent of 6 grams 2 micrograms per day of Ra-226 and Ra-228.
Opponents of nuclear often point out that aged spent fuel becomes more dangerous as it decays. When, for example, U-238 first goes into the landfill, it will be clean of any daughter products. What they are referring to is the slow build in those daughters, most importantly, Lead-214 and Bismuth-214, both of which are photon emitters. But with radiation it’s always about the numbers. The concentration of the daughters will build over a million or so years until they are in secular equilibrium, which is a fancy way of saying for every decay of U-238, there will be a decay of each daughter. In the case of Pb-214, that will be when there are 10.2 nanograms of Pb-214 per ton of uranium. The Bi-214 concentration will be slightly less, 7.6 nanograms per ton. At that point, the U-238 will be as dangerous as it was when we pulled it out of the ground.
But wait another few tens of billions of years and all the uranium will have become lead. So maybe they have a point.



Can't find ORNL report https://www.ornl.gov/search/node?keys=TM-13170